Your basket is currently empty!


• Symposium brings together global leaders in immunogenicity to connect with China’s rapidly evolving pharmaceutical development landscape
• Speakers include thought leaders in biotherapeutics, complex peptide drug developers, and regulatory authorities
• In-person event taking place at Oriental Riverside Hotel, Shanghai, 13-14 April 2026
OXFORD, UK – 21 Jan 2026 – ProImmune Ltd, a global leader in immunological reagents and services, announced the launch of Mastering Immunity 2026 – Shanghai, an inaugural symposium in China providing practical, real-world guidance to help drug developers manage immunogenicity risk. Taking place at the Oriental Riverside Hotel in Shanghai, the event will convene leading scientists, industry executives, and regulatory professionals from around the world.
Immunogenicity, the potential for biologics to trigger unwanted immune responses, remains a decisive factor in determining safety, efficacy, and regulatory and commercial success. When immunogenicity is poorly understood or assessed inadequately, development programs face clinical failure, costly redesigns, and significant delays. In some cases, these risks can lead to the loss of entire programs, representing financial impacts in the hundreds of millions or even billions of dollars, as well as extended timelines required for additional studies or reformulation.
Presentations will cover modality-specific insights based on real world examples for emerging therapeutic platforms and perspectives on evolving global regulatory expectations presented by speakers from leading companies and regulators with experience of bringing successful drugs to market, including:
Dr. Zuben Sauna (Director, Center for Biologics Evaluation and Research, FDA)
Dr. Sophie Tourdot (Immunogenicity Sciences Lead, Pfizer)
Dr. Balaji MR (Vice President and Head – Non Clinical, Dr. Reddy’s Laboratories)
Dr. Daniela Verthelyi (Former Chief, Laboratory of Immunology, Office of Biotechnology Products, FDA)
Dr. Tim Hickling (Former Head of Immunosafety, Roche, Quasor)
Dr. Kanthikiran Varanasi (Global Head of Clinical Affairs, Galencium)
Andreas Hollenstein (Senior Principal Scientist, Immunosafety, Roche)
Dr. Nitinkumar Dobaria (Associate Director, Injectables Product Development, Lupin)
Dr. Anuj Saini (Global Head-Global Clinical Management, Dr Reddy’s Laboratories)
Prof. Yoonjoo Choi (Professor, Department of Microbiology and Immunology, Chonnam National University Medical School)
Dr. Juhao Yang, Toxicology Project Leader, China Innovation Center of Roche shared: “This meeting is a pivotal moment, bringing together global leaders to tackle the urgent challenge of immunogenicity risk and drive better patient outcomes. This is a must-attend event for anyone committed to shaping the future of biotherapeutics.”
Dr. Nikolai Schwabe, Chief Executive Officer, ProImmune, added: “Licensing activity and clinical trials are at an all-time high in China’s biopharmaceutical industry, but the regulatory landscape is still evolving. By connecting immunogenicity leaders from around the world to share experiences including development and regulatory best practice, ProImmune is playing a critical role in helping developers manage the risk around immunological effects and ultimately accelerate the development of successful therapeutic products.”
For event details and registration, including early-bird, visit: https://www.proimmune.com/mastering-immunity-2026-shanghai
Oxford, UK – 30 October 2025 – ProImmune Ltd, a global leader in life science reagents and services, today announces the launch of its new ProVE® SL Self-Loading MHC Class I Monomers. The novel reagents for antigen-specific CD8+ T cell detection are designed to offer researchers unprecedented flexibility, speed, and MHC allele-coverage, with over 50 HLA alleles available.
Supplied as highly robust, empty, biotinylated MHC Class I molecules, ProImmune’s ProVE SL Monomers offer an extended allele range including HLA-A, HLA-B, HLA-C and non-canonical alleles, as well as mouse H-2. The ProVE SL platform allows researchers to load their own peptides and flexibly combine monomers with a wide range of avidin or streptavidin conjugates—including fluorescent, barcoded, or mass-cytometry reagents—to easily create tetramers or surface-bound complexes. This flexibility enables faster experimental setup, reconfiguration of panels, expanded donor-cohort coverage, including less common alleles, and integration with advanced flow and mass cytometry or single-cell RNA sequencing workflows.
T cell immunology, epitope mapping and immune-monitoring workflows increasingly demand reagents that permit rapid customisation across diverse HLA contexts. The ProVE SL monomers address key limitations of conventional pre-loaded MHC monomers, which often restrict peptide choice, require complex loading and exchange steps, or cover only frequent alleles. By enabling self-loading of peptides, the ProVE SL platform empowers immunologists to adapt their reagents more quickly to new antigens, for example for infectious diseases, cancer neo-antigens, autoantigens, and measuring vaccine responses and to study donor cohorts with rarer HLA types.
“With the launch of our new ProVE SL monomers, we are offering researchers the ability to customise MHC monomer reagents at unprecedented speed and breadth,” said Dr. Nikolai Schwabe, CEO at ProImmune. “This flexibility will accelerate epitope mapping, immune-monitoring and translational across research programmes, including in infectious disease and oncology. ProVE SL Monomers are a natural extension to our market-leading product ranges in MHC Class I/II monomers, multimers, and immunology services, as well as our extensive peptide synthesis capability”.
The ProVE SL Self-Loading MHC Class I Monomers are available now through ProImmune’s catalog and distributor network. For more information, visit: https://www.proimmune.com/prove-sl-self-loading-mhc-class-i-monomers
To order online, visit: https://www.proimmune.com/product-category/mhc-multimers/self-loading-mhc-class-i-monomers

• Move follows ProImmune’s recent acquisition of Oasis Park a 70,000 sq ft Business and Light Industrial Park in Oxford, UK
• Increased operational capacity enables accelerated development of ProImmune’s REVEAL® Immunogenicity System and Ankyron® target binding technologies
Oxford, UK – 22 October – ProImmune Ltd, a global leader in life science reagents and services, today announced the opening of its new global headquarters building at Oasis Park, Oxford, enabling significant expansion of its operations and capacity to serve its global client base.
Located within Oxford’s renowned bioscience and innovation ecosystem, the 70,000 sq ft Oasis Park is already home to six existing tenants. ProImmune’s new 18,000 sq ft main headquarters facility represents a substantial investment in state-of-the-art laboratories, providing ProImmune with the space and infrastructure to accelerate innovation in the REVEAL® Immunogenicity System, its suite of assay services to help understand immune responses to vaccines and therapeutic products, and the expansion of the Company’s revolutionary Ankyron® target binding reagent platform. Ankyrons are small, recombinant, target binding ankyrin repeat proteins, developed by ProImmune to overcome challenges in in antibody research. The high-specificity, sensitivity, and reproducibility of Ankyrons offers a cost-effective alternative to generating custom antibodies.
“Our new global headquarters at Oasis Park, Oxford, are a reflection of ProImmune’s commitment to scientific excellence in immunology and global one-health biology,” said Dr. Nikolai Schwabe, Chief Executive Officer of ProImmune. “The investment into the park, and into our new flexible laboratory and office facilities, strengthens our ability to deliver world-class products and services, supporting our partners to develop safer and more effective therapies for patients around the world.”
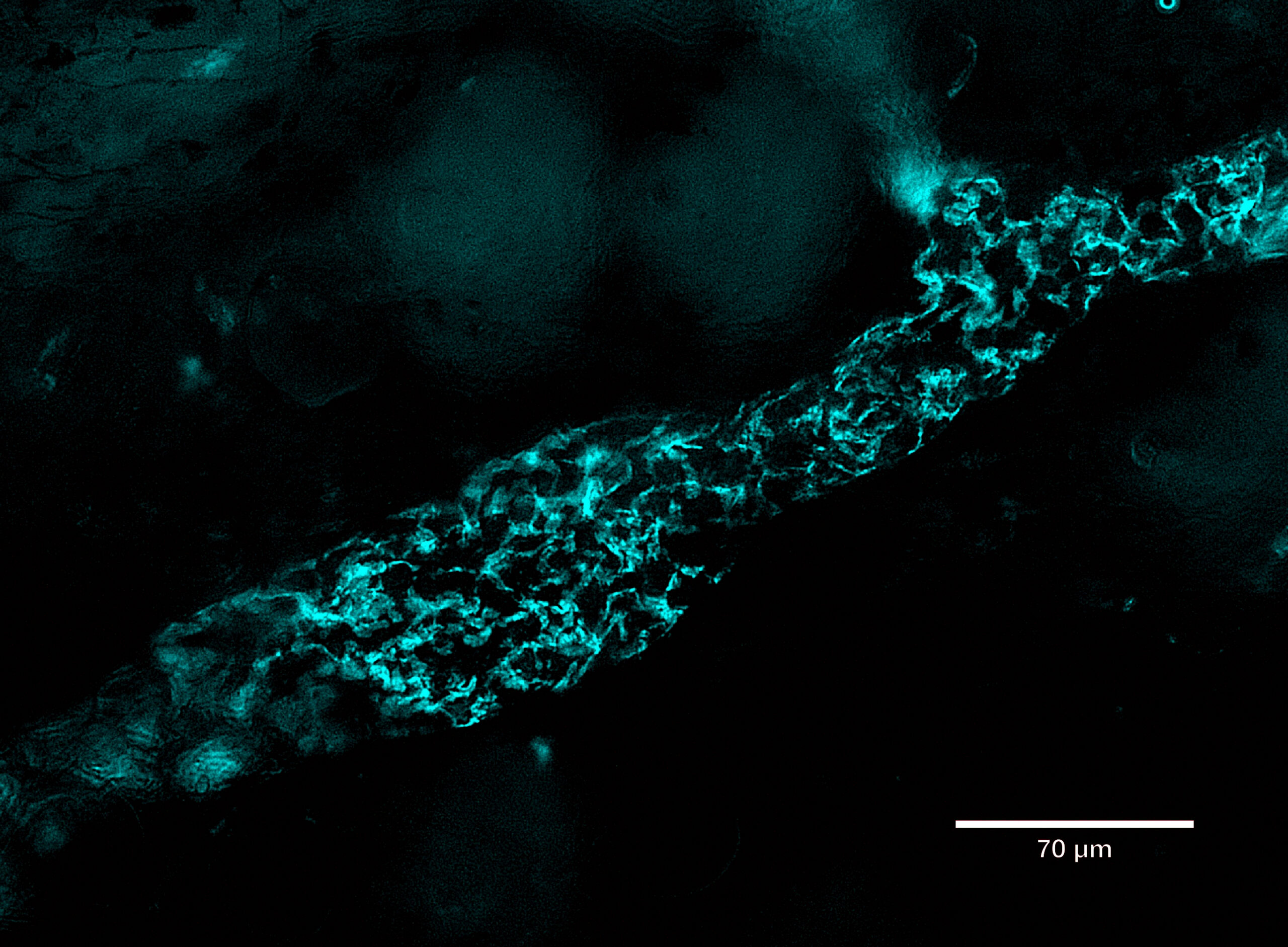
ProImmune is spotlighting the capabilities of its next-generation recombinant binding reagent, Ankyron® clone CJ30451, in a recent visualization of LYVE1, a key lymphatic vessel marker.
Using fluorescence microscopy, LYVE1 was detected in cyan at the overlapping flaps of endothelial cell junctions in skin tissue. The breakthrough lies in the ultra-compact size of Ankyrons—approximately one-tenth the size of traditional monoclonal antibodies (~15 kDa)—which allows them to navigate and label tight cellular junctions with high precision. This enables detailed mapping of endothelial structures previously difficult to access.
LYVE1, beyond its structural role, serves as a crucial gatekeeper for immune surveillance and cancer metastasis, binding to hyaluronan on immune and tumor cells to facilitate their entry into the lymphatic system.
The image was captured using ProImmune’s trusted DiscoverEcho microscope platform, underscoring the synergy between advanced imaging and molecular targeting tools.
Importantly, Ankyrons are 100% animal-free, offering a sustainable and ethical alternative for researchers in immunology, oncology, and vascular biology.
Learn more about Ankyrons here.

A strategic partnership between The Pirbright Institute and ProImmune aims to develop target-binding reagents that significantly accelerate research in animal health.
Ankyrons™ are small, recombinant, target binding ankyrin repeat proteins developed by ProImmune. They are directly selected in vitro from a trillion-clone library in ribosome display and, like antibodies, can bind with high affinity to almost any target.
Due to their superior properties compared to antibodies, Ankyrons™ open new avenues for scientists to study cell behaviour, offering an alternative to traditional research antibodies. The high specificity, sensitivity and reproducibility of Ankyrons™ means they can be broadly applied in biomedical science and can be targeted against all structural proteins as a cost-effective alternative to generating custom antibodies.
In this collaboration, ProImmune is aiming to establish new Ankyrons™ for a range of crucial veterinary research applications where antibodies were previously unavailable. ProImmune has already generated and provided new Ankyrons™ to Pirbright for further study and validation, including for a broad range of bovine, porcine, avian and mosquito protein targets, as well as targets for significant animal diseases including Foot and Mouth Disease and African Swine Fever – all areas that have been underserved by traditional antibody technology.
Pirbright’s Director of Research, Professor John Hammond, said: “The collaboration brings mutual benefits to address global animal health challenges. Multiple research groups across the Institute have worked with ProImmune to develop novel Ankyrons™ that address targets from across the tree of life including mammals, insects and viral proteins.”
Dr Linda Tan, Chief Scientific Officer, ProImmune, said: “Ankyron™ technology gives researchers a better handle on biology. In this collaboration we have already demonstrated that novel Ankyrons™ can be provided rapidly to dozens of targets in areas totally unaddressed by existing research tools. We believe that over time Ankyron™ technology will have a profound impact in the wider study of biology across species and the results from this collaboration are already testament to this.”
ProImmune and the Pirbright Institute hope to make significant strides in addressing global health challenges through the development of this cutting-edge research technology that will be available to researchers world-wide, studying animal physiology and disease.

used by team at GSK to study mRNA vaccines that can protect against several Flu strains
Magini D. et al., PLOS One (2017)
Self-Amplifying
mRNA Vaccines Expressing Multiple Conserved Influenza Antigens
Confer Protection against Homologous and Heterosubtypic Viral
Challenge
http://dx.doi.org/10.1371/journal.pone.0161193
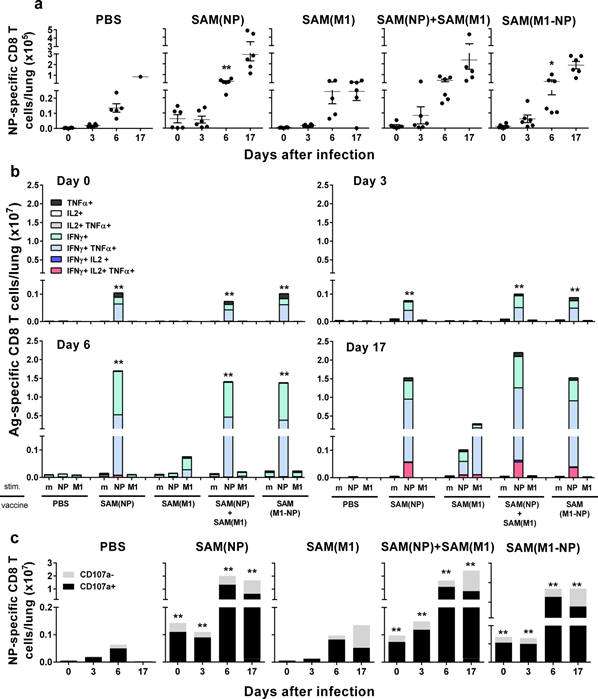
Figure:
NP-specific
CD8+ T-cell responses in lungs after influenza
challenge. BALB/c mice
were immunized i.m. twice, 8 weeks apart, with PBS, 0.1 μg of
self-amplifying mRNA (SAM®) vectors SAM(NP), SAM(M1),
SAM(M1-NP), or with 0.2 μg of SAM(NP)+SAM(M1). Four weeks after
the second immunization, mice were infected with PR8 virus.
NP-specific CD8 T cells recruited in the lungs after the
infection were characterized by flow cytometry. (a) Numbers of
NP-specific CD8+ T cells determined using
Pro5® MHC Pentamers. Data are from individual mice (depicted as dots), while solid lanes
indicate the mean±SD. (b) Cumulative frequency of Ag-specific,
cytokine-secreting CD8+ T cells, indicated as
absolute number per lung. The color code represents the
different combinations of cytokine produced by the respective
cells after in vitro stimulation with medium (m), NP147-155
peptide (NP), or M1 peptide pool (M1), as indicated. (c)
Absolute number of NP-specific CD8+ T cells positive
(black bar) or not (grey bar) for CD107a. Data derived from two
independent and merged experiments. Statistical analyses were
performed using the Mann-Whitney U test. *p<0.05; **p<0.01
compared to the PBS-treated group.
http://dx.doi.org/10.1371/journal.pone.0161193.g006
Abbreviated abstract:
Current hemagglutinin (HA)-based seasonal influenza vaccines induce
vaccine strain-specific neutralizing antibodies that usually fail to
protect against mismatched circulating viruses. Inclusion of
conserved proteins such as the nucleoprotein (NP) and the matrix
protein 1 (M1) can increase effectiveness by eliciting
cross-reactive T-cells. However, efficient priming of T-cell
responses requires the right delivery system. Here we show novel
self-amplifying mRNA (SAM®) vectors expressing influenza
NP (SAM(NP)), M1 (SAM(M1)), and NP and M1 (SAM(M1-NP)) delivered
with lipid nanoparticles (LNP) induce robust polyfunctional CD4 T
helper 1 cells, while NP-containing SAM also induced cytotoxic CD8 T
cells. Robust expansions of central memory (TCM) and
effector memory (TEM) CD4 and CD8 T cells were also
measured. An enhanced recruitment of NP-specific cytotoxic CD8
T cells was observed in the lungs of SAM(NP)-immunized mice after
influenza infection that paralleled with reduced lung viral titers
and pathology, and increased survival after homologous and
heterosubtypic influenza challenge. We show for the first time that
the co-administration of RNA (SAM(M1-NP)) and protein (monovalent
inactivated influenza vaccine (MIIV)) was feasible, induced
simultaneously NP-, M1- and HA-specific T cells and HA-specific
neutralizing antibodies, and enhanced MIIV efficacy against a
heterologous challenge.
Pro5® MHC Pentamer

Pro5® MHC Class I Pentamers are the market leading product for detecting antigen-specific T cells, you can count on consistent performance backed up by unrivalled customer service.
To ensure uniformity and consistency, each Pentamer is purified four times before being subjected to rigorous quality control acceptance testing.