Your basket is currently empty!
Optimizing-Cell-Mediated
Case Study: Optimizing Cell Mediated Immunity assay methodology using ProImmune Pro5® MHC Pentamers
| Afonso G. et al. (2010). Critical parameters in blood processing for T-cell assays: Validation on ELISpot and tetramer platforms. Journal of Immunological Methods. 359: 28-36. [PubMed ID 20641145]Monitoring antigen (Ag)-specific T cell responses in peripheral blood mononuclear cells (PBMC) forms a cornerstone of the work of many investigators, from those interested in understanding disease pathogenesis to those developing therapeutic vaccines. |  |
Georgia Afonso (pictured) and her co-workers took a systematic approach to analyzing the differing methods that may be utilized for blood collection, storage and processing, and their effects on commonly used cell-mediated immunity (CMI) assays. As readouts they employed two assays, IFN gamma ELISPOT and flow cytometry staining with a ProImmune Pro5® MHC Pentamer (A*02:01/GILGFVFTL, Influenza MP58-66). They found that differences in the handling of blood samples make a substantial difference to the outcomes of these assays and in an assay-specific fashion.
Firstly, the effects of blood drawing devices (vacuum driven or syringe), of gentle agitation of the blood sample post-draw, and of isolating PBMC via density gradient centrifugation using either standard or membrane (Leucosep®) tubes, were all compared. None of these parameters significantly affected the ELISPOT or Pro5® Pentamer staining results for cells analyzed 3 hours post-draw.
The team next moved to investigate the effect of overnight storage on assay performance. The storage of blood with gentle agitation for 18 hours before processing led to a significant decrease in the numbers of IFN gamma-secreting cells detected by ELISPOT, and separately, improved ELISPOT responses were obtained using stored PBMC isolated using standard as opposed to membrane tubes. In terms of Pro5® Pentamer staining, overnight storage enhanced the assay results. Those samples stored for 18 hours, rather than 3 hours, before processing (and particularly those stored with agitation) exhibited increased frequencies of influenza-specific Pentamer-positive cells (Figure 1).
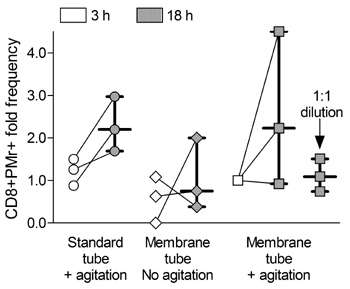 |
Figure 1: Differences in CD8+ Pentamer+ cell frequencies in blood stored for 3 h (white symbols) or 18 h (grey symbols) after drawing and processing as indicated. Each symbol represents one sample, with lines connecting values obtained at 3 h and 18 h from the same starting sample. Bars indicate the median± (interquartile) range of the corresponding distribution. Values are normalized to the “membrane tube+agitation” condition at 3 h, and refer to 3 independent experiments. |
Storage of fresh blood has previously been found to cause contamination of PBMC with granulocytes, and Afonso et al. similarly found noticeable granulocyte contamination present after 18 hours of storage, but showed that gentle agitation of the samples, and dilution of the blood 1:1 with RPMI medium, ameliorated this.
Finally, the team investigated the effects of PBMC washes in varying concentrations of human serum. They found that statistically significant increases in PBMC yield accompanied washing in serum, at concentrations of up to 10%. Again the researchers assessed the performance of these cells in both ELISPOT and Pro5® Pentamer staining assays. While performance in ELISPOT was unaffected by the washes, Pro5® Pentamer staining was decreased in PBMC preparations which had been washed in higher concentrations of serum (Figure 2). It is therefore necessary to strike a balance between cell recovery, and performance in an MHC Pentamer staining assay.
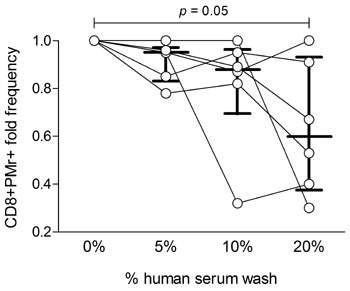 |
Figure 2: CD8+ Pentamer+ cell frequencies from PBMCs washed with different human serum concentrations in RPMI medium. Each symbol represents one sample, with lines connecting values obtained from the same starting sample with different human serum concentrations. Bars indicate the median± (interquartile) range of the corresponding distribution. Results are normalized to samples washed without human serum, and refer to 6 independent experiments. |
Taken together, the results of Afonso et al. highlight the sensitivity of performance of CMI assays to the methods used to isolate and store PBMC prior to experimentation. The observation that different conditions may be optimal for different assay types (here ELISPOT and Pro5® Pentamer staining) underlines the importance of pilot studies to optimize each assay prior to large-scale experiments. In this study, IFN gamma ELISPOT and Pro5® Pentamer staining with the Influenza MP58-66 Pro5® Pentamer were used as exemplars, but it should be borne in mind that the optimal conditions for ELISPOT for alternative cytokines, or staining with other Pentamers, may well differ from those described by Afonso et al.
At ProImmune, we are experienced in assisting customers with the planning of their experiments, and are always happy to recommend best-practice strategies for the design of feasibility studies, whether these are performed by the customer or by ProImmune as part of our Cellular Analysis Services.
This work was carried out in the DeAR Lab Avenir, Inserm U986, Paris, France, www.dearlab.org
Images Copyright (2010), with permission from Elsevier
