Your basket is currently empty!
Killer T Cells Trained for Long-lasting Anti-Cancer Effects
Killer T Cells Trained for Long-lasting Anti-Cancer Effects
Establishment of Antitumor Memory in Humans Using In Vitro-Educated CD8+ T Cells
Butler, M. et al. (2011). Establishment of antitumor memory in humans using in vitro-educated CD8+ T cells. Science Translational Medicine. 3(80): 80ra34. [PubMed ID: 21525398]
Cancer immunotherapy is an exciting area of research, designed to harness the power of the body’s own immune system to fight the disease. For Marcus Butler and his colleagues at the Dana Farber Cancer Institute, the dream would be to generate a library of killer T cells that could be used to target and destroy cancer in patients. Recently published work from this team gives hope that this dream could become a reality in the next 5-10 years following their research on melanoma.
Melanoma is a highly malignant tumor of the pigmented cells found in skin, and in its advanced stage has a poor prognosis – the median patient survival time is less than a year. In melanoma, and other cancers where the tumor antigens are well-characterized, there has been a burgeoning interest in adoptive T cell transfer as a therapy. With this strategy, CD8+ cytotoxic T cells specific for tumor antigens are isolated (by flow-cytometry sorting the bulk CD8+ population and preferentially expanding the antigen-specific population through peptide stimulation), and then returned to the patient. The tumor-specific T cells traffic to the tumor and destroy target cells, controlling or even eliminating the tumor. Since the T cells are patient-derived, there is minimal toxicity, so the therapy remains a very attractive option. However, the effectiveness of adoptive cell transfer can be improved upon. A major limitation is the lifespan of transferred T cells – without repeat rounds of treatment and manipulation of the host immune system such as lymphodepletion, the transferred cells persist for only a week on average, and the therapeutic benefit is short-lived.
Generating the best cells for adoptive transfer
Marcus Butler and his co-workers thought laterally about this problem, and their results, published in Science Translational Medicine, show that they are on the right track to solving it. For adoptive cell transfer to work successfully, the transferred antigen-specific T cells need to persist for longer. Their idea was to generate effector memory CD8+ T cells (with a surface marker phenotype CD45RA-, CD45RO+, CD62L+/-), possessing both anti-tumor specificity and the longevity associated with an effector memory phenotype.
The team have previously published data [PubMedID: 17363542] on the generation of artificial antigen presenting cells (aAPC). Using aAPC and IL-15/IL-2 cytokine treatment of tumor specific T cells isolated from patients, they are able to expand a population of antigen-specific effector memory T cells that survive well in vitro. The next step was a proof-of concept study to show that these cells could persist and function in vivo.
They recruited 9 patients with end-stage melanoma, and isolated and expanded CD8+ T cells specific for the melanoma antigen MART-1 (ELAGIGILTV) using their aAPC system. MART-1-specific cells were infused into patients twice, at a 35-day interval, without any other manipulation of host immunity.
Increased numbers of tumor-specific cells
Using staining with MART-1 Pro5® MHC Class I Pentamer to monitor responses, increased numbers of MART-1-specific cells were observed at 2 and 3 weeks after treatment, and in 3 patients, increased MART-1 cell numbers were apparent up to a year post-infusion.
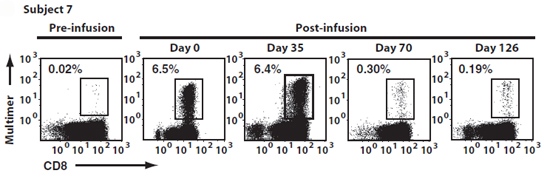
Figure 1: Adoptive transfer induced sustained increases in the frequency of circulating MART1-specific CD8+ T cells. The frequency of MART1-specific T cells was determined by MART1 Pro5® Pentamer staining of circulating CD8+ T cells before and after infusion. Representative A*02:01/ELAGIGILTV Pentamer staining for subject 7 is shown. Day 0 and 35 analyses were performed on blood samples drawn 30 min after infusion of CTL grafts. Data reproduced with permission from Science Translational Medicine.
The transferred cells were followed by monitoring the phenotype of MART-1-specific CD8+ T cells, and could be distinguished from endogenous MART-1 specific cells through their continued expression of memory markers. Their functionality was confirmed by IFN-gamma ELISpot and also by applying MART-1 peptide to patient to induce a delayed-type hypersensitivity reaction. By both of these measures, anti-MART-1 T cell activity was enhanced after treatment. Importantly, the infused T cells were able to traffic to tumor sites; this was demonstrated by tumor biopsy (an increase in tumor infiltrating lymphocytes was apparent) and by sequencing the T cell receptors of the tumor-infiltrating lymphocytes and comparing these to the sequences known to be present in the infused cells.
Clinical Responses
70 days after treatment, one patient had achieved complete remission, four were stabilized, three had progressive disease, and one died before receiving a second infusion of cells. From such a small patient cohort, it is not possible to draw firm conclusions, but the results are very promising.
Following the adoptive cell therapy trial, five patients subsequently received Ipilimumab® as a CTLA-4 blockade. The treatment ameliorated the condition of most of those who received it, and one patient achieved almost complete resolution of her disease. This is of note because the response rate for Ipilimumab® treatment overall is low, at around 16%, and there exists evidence that CTLA-4 blockade can induce expansion of adoptively transferred CD8+ T cells. Butler et al suggest that the efficacy of their aAPC-induced memory effector anti-tumor cell therapy would be further enhanced by including CTLA-4 blockade in a treatment regime. Combining their enhanced adoptive cell transfer protocol with existing treatments like this could, the investigators suggest, substantially improve prognosis for those who are seriously ill with melanoma.
