Your basket is currently empty!
Defining-autoantibody
Case Study: Defining autoantibody binding to citrullinated target proteins in Rheumatoid Arthritis using Peptide Arrays
Klareskog, L., et al. (2008). Immunity to Citrullinated Proteins in Rheumatoid Arthritis. Annual Review of Immunology. 26: 651-675. PubMedID:18173373
Professor Lars Klareskog and his team at the Karolinska Institute in Stockholm, Sweden, have a long-standing research interest in Rheumatoid Arthritis (RA). RA onset is associated with MHC class II–dependent activation of adaptive immunity. A variety of proteins (such as vimentin, alpha-enolase, type II collagen, and fibrinogen) undergo posttranslational modification of arginine to citrulline over time, and a working hypothesis is that this may be aggravated by smoking or bacterial infection. Autoantibodies towards these modified proteins may complex with their target citrullinated proteins as part of a multistep process for RA development.
Increasing the number of B cell epitopes on candidate autoantigens is central when dissecting this complex disease and with that intent researcher Lena Israelsson turned to ProImmune’s B cell Epitope Mapping Service.
ProImmune synthesized a series of novel and previously identified peptides from RA-associated proteins in both their native and their citrullinated variants, then spotted them onto multiple ProArray® slides for screening. Sera collected from RA patients were assayed for binding to the paired native and citrullinated peptides. Across the set of patient samples, differences were apparent between citrullinated peptides and their unmodified counterparts, as shown in figure 1, below.
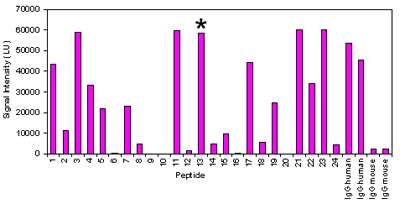
Figure 1: ProArray® Results from a test serum sample for binding to 12 peptide pairs (native and citrullinated) from RA-associated proteins. Asterisk indicates result from one citrullinated fibrinogen peptide sequence (peptide 13), shown next to result from the native sequence (peptide 14).
For each of the peptide pairs screened, it was evident that the patient antibodies bound more strongly to the citrullinated variant than the native peptide. ProImmune’s peptide array based mapping is sufficiently sensitive to highlight these differences in binding. While further validation work is required, provisionally, these results support the hypothesis that citrullinated epitopes could be important for the development of RA. The data generated by ProImmune in a short space of time, helped shortlist some of the novel peptides for further studies at the Karolinska Institute.
This work was carried out at ProImmune and at the Karolinska Institute.
