Your basket is currently empty!
Case Study: Pro5 MHC Class I Pentamers used to evaluate vaccine safety
Case Study:
Pro5® MHC Class I Pentamers used to evaluate vaccine safety
Elliott, SL. et al. (2008). Phase I trial of a CD8+ T-cell peptide epitope-based vaccine for infectious mononucleosis. J Virology. 82(3): 1448-1457. [Pubmed ID: 18032491]
A Phase I clinical trial by Elliott et al. investigated the potential safety of a CD8+ T cell peptide-epitope vaccine against infectious mononucleosis (IM). The vaccine comprised a single EBV EBNA3A derived peptide, FLRGRAYGL, mixed with tetanus toxoid, and was delivered subcutaneously to B*08:01-positive, EBV-seronegative volunteers.
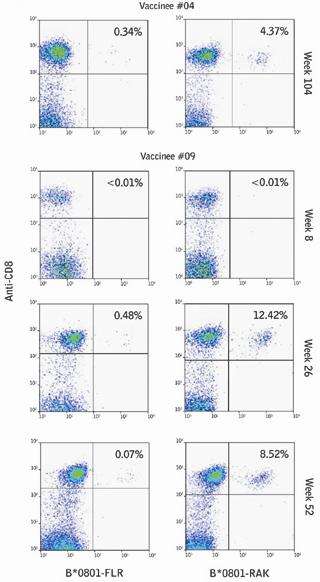 |
EBV-specific Pro5® MHC Class I Pentamers, B*08:01/FLRGRAYGL and B*08:01/RAKFKQLL, were used to monitor antigen-specific responses post-vaccination in order to ensure that the vaccine was safe in the event of a subsequent EBV infection, and to verify whether or not the immune response was limited to just the single epitope.Rapid recovery from IM symptoms has been correlated with broad T cell reactivity to multiple CD8+ T cell epitopes, whereas prolonged illness was associated with a narrowly focused response. Analysis of FLR- and RAK-specific responses in two vaccinees who seroconverted asymptomatically within 2 years of the trial, showed responses similar to those seen in healthy, seropositive individuals or subjects with infectious mononucleosis.
There was no evidence that vaccinated individuals were predisposed to abnormally high FLR-specific responses after EBV seroconversion, demonstrating that the single epitope vaccine did not lead to an immunodominant response to the single FLRGRAYGL epitope. The study concluded that the single epitope vaccine was well tolerated and immunogenic in subjects, and did not predispose patients to disease following EBV infection. |
The figure shows the CD8+ T cell response of vaccinees after EBV seroconversion. PBMCs from vaccinees (no. 9 and 4) collected at the indicated time points post-vaccination were assessed by flow cytometry using FLR-specific (left) and RAK-specific (right) Pentamers and anti-CD8 monoclonal antibody. The percentage of CD8+ T cells that are Pentamer-positive is indicated in the top right corner of each panel.
Copyright 2008 American Society of Microbiology
