Your basket is currently empty!
Multimer Flow Cytometry
Multimer Flow Cytometry
The detection of events as rare as 0.02% of total T cells requires both the design of suitably controlled experiments and a well-maintained flow cytometer. By following the protocols supplied with our products carefully, you should achieve successful staining. Below are suggestions to help you troubleshoot, should unsatisfactory staining be obtained. Please also consult the protocol optimization pages, or Pro5® MHC Pentamer handbook.
For expert guidance, the technical support team at ProImmune are all Ph.D-qualified Immunologists, with years of lab experience between them.
| Cell Preparation | Non-Specific Staining |
| Lack of Staining | Further Reading |
Cell Preparation, Instrument Set-Up and Compensation
In order to achieve successful Pentamer staining, the following points should be noted:
Cell Preparation:
Care should be taken with preparation of cells to maximize recovery and obtain a sufficient quantity of viable cells for staining. An excess of dead or dying cells will result in poor quality staining and increased background.
Instrument Set-Up:
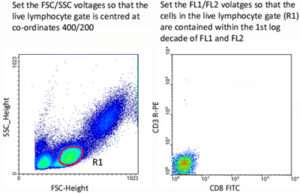
Flow cytometer instrument settings should be determined empirically for each different cytometer using samples of unstained cells and single-color controls. Forward scatter (FSC) and side scatter (SSC) voltages should be set using unstained cells so that the live lymphocyte population lies on scale (normally positioned at co-ordinates 400 and 200 respectively). Set a gate upon the live lymphocyte population, then set the voltages for each individual fluorescent channel so that the live cells are contained within the first log decade of each fluorescent channel.
Compensation:
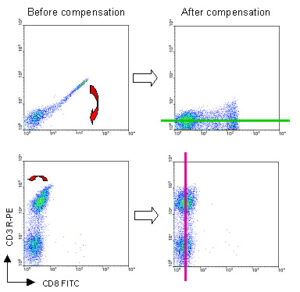
Use single-color stained controls to adjust compensation settings for all the fluorescent channels required for the experiment. Due to overlapping emission spectra, some fluorescence from one fluorochrome may be detected in another channel. If the overlap is not corrected by compensation, the data will include the signal from each of the fluorochromes that has been ‘seen’ by the inappropriate detector. Set the compensation so that the center of the positively stained cell population lies in line with the center of the negative cell population and parallel with the appropriate axis.
Back to top
Non-Specific Staining – High Background
Non-specific staining is defined as events seen in the CD8-negative, Pentamer-positive quadrant of antigen-specific cells, or in the CD8-positive, Pentamer-positive quadrant of negative control cells.
Common Reasons for Non-specific Staining |
|
| Pentamer was not centrifuged prior to staining | Pro5® MHC Pentamers and Fluorotags should be centrifuged at 14,000 rpm for 10 minutes prior to staining to remove any aggregates before each use of the Pentamer. |
| Too much Pentamer for the number of cells | One test of Pentamer should stain 1-2 x 106 antigen-specific cells. However, it is recommended that the Pentamer is titrated prior to use. Carry out a range of doubling dilutions from 1 test per 1 x 106 cells down to 1/16 test per 1 x 106cells. This will solve most cases of non-specific Pentamer staining. |
| The temperature used for staining was incorrect | For Pentamers, try comparing staining at 22ºC (room temperature), to staining at 4ºC. We do not recommend staining at 37ºC since this can yield considerable non-specific staining. |
| Incubation time with Pentamer was too long | Pentamer staining for 10 minutes at room temperature (22ºC) is sufficient. However, if staining at 4ºC the incubation time may be increased to 30 minutes. Incubation for longer periods may increase background. |
| Insufficient washing between staining steps | Cells should be washed with a 10x volume of buffer between staining steps (some specificities may require 2 washes between steps). |
| The stock Pentamer has not been stored according to instructions | Labeled Pentamers and Fluorotags should be stored consistently at 4ºC in the dark. Unlabeled and biotin-labeled Pentamers may also be stored at -80ºC. |
| B cells are interfering with the staining | Cells may be co-stained with anti-CD19 antibody to gate out B cells (see next section). Alternatively, anti-CD19 magnetic beads could be used to remove B cells prior to staining. |
| Buffer contains phenol red | If using RPMI-1640, HBSS or DMEM as a medium in which to stain cells it is recommended that this does not contain phenol red, which can contribute to autofluorescence and thus appear as non-specific staining on analysis plots. |
| Pentamer binds non-specifically to the cells | If cells in the sample are in a sub-optimal condition the Pentamer may bind non-specifically to the cell surface. This can be eliminated by first blocking the cells with 4 µg/ml pure IgG or 10% human or mouse serum. The Pentamer can then be added directly onto the sample + blocking reagent. |
Back to top
Elimination of Non-Specific Staining in Splenocyte or Blood Samples
Background (non-specific) staining is sometimes observed when performing flow cytometric analysis of human and murine samples, particularly in the CD8-negative, Pentamer-positive quadrant. This problem is largely caused by non-specific staining of B cells, and can be reduced or eliminated by co-staining with anti-CD19 antibody and gating on the CD19-negative cells when performing analysis. Alternatively, anti-CD19 magnetic beads could be used to remove B cells prior to staining.
Application note: Elimination of non-specific staining (PDF)
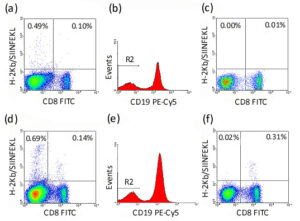
The figure below demonstrates the effect of gating out B cells from murine splenocytes. 1 x 106 naive C57BL/6 (a)-(c) or SIINFEKL-immunized C57BL/6 (d)-(f) splenocytes were incubated with 1 test unlabeled H-2Kb/OVA-specific Pro5® MHC Pentamer (SIINFEKL) for 10 minutes at room temperature (22°C). Cells were washed, then further incubated with 1 test Pro5® R-PE Fluorotag, 1 test anti-mouse CD8-FITC (clone KT15) and 1 test anti-mouse CD19-PECy5 (clone 6D5) for 30 minutes at 4°C. In (a) & (d), the live splenocyte population shows considerable non-specific staining in the CD8-negative, Pentamer-positive quadrant; (b) & (e) A region R2 is set upon the CD19-negative cells; (c) & (f) The live splenocyte population is re-gated to show only events within R2 (which excludes B cells), and the non-specific staining has now been eliminated. This illustrates that a substantial majority of the non-specific staining can be attributed to B cells.
Lack of Staining
Common Reasons for Lack of Staining |
|
| The antigen-specific population is not present | In addition to staining by flow cytometry, it is advisable to use an alternate method to verify the presence or absence of an antigen-specific population. Functional assays such as the study of cytokine secretion using ELISpot, intracellular cytokine staining or a cytokine secretion assay are recommended. |
| Using an incorrect MHC Pentamer for the cell type/species | Check that your samples are positive for the MHC allele/tissue type of the MHC Pentamer you are intending to use and that the antigen you intend to investigate is only presented through this MHC allele. |
| Too little Pentamer for the number of cells | One test of Pentamer should stain 1-2 x 106 antigen-specific cells. However, it is recommended that the Pentamer is titrated prior to use. Carry out a range of doubling dilutions from 1 test per 1 x 106 cells down to 1/16 test per 1 x 106cells. |
| The antigen-specific population is below the threshold of detection | The threshold of detection of antigen-specific events depends on the type of cells used and their method of preparation. For human PBMCs the threshold is typically around 0.02%. Detecting a smaller population is possible but full protocol optimization may be required. An improvement in results may be achieved by enriching the population of T cells. This may be done using magnetic beads or by stimulation of cells in culture using peptides or antibodies.Please note that mouse immunisation protocols need to be optimized to produce sufficient immune responses. Several rounds of immunization, with appropriate adjuvants, may be required to achieve a response that exceeds the threshold of detection. |
| Cells were (re)stimulated in vitro without subsequent resting | In vitro stimulation of T cells in culture will usually leads to a down regulation of their T cell receptor (TCR) . Cells need to be rested for a sufficiently long period after such in vitro stimulation in order for TCR expression to recover on the cell surface so that cells can be stained successfully. Where cells have been stimulated in vitro, we recommend resting cells for at least one week after the stimulus has been removed from cell culture before Pentamer staining. |
| Insufficient events acquired | When looking at rare populations, such as antigen-specific T cells, it is important to collect as many cells as possible; if expecting a result of only 0.1%, a sample size of 500,000 cells would show 500 dots on the plot. It is important to bear in mind the expected frequency of events to be collected and adjust the starting sample size accordingly. |
| Use of sub-optimal anti-CD8 antibody clone | The anti-CD8 antibody used for co-staining should be selected carefully as some clones interfere with Pentamer binding to the TCR. ProImmune has carried out extensive testing of Pentamers with different anti-CD8 clones and staining conditions. Clones LT8 (human) and KT15 (mouse) are recommended as they do not block binding of Pentamers to the TCR. Both of these antibody clones are available from ProImmune. |
| Reagent has expired | ProImmune guarantees all Pro5® MHC Pentamers for 6 months from delivery when stored according to instructions. All ProVE® MHC Pentamers are guaranteed for 3 months from delivery when stored according to instructions. From our regular stability testing we have determined that Pro5® and ProVE® MHC Pentamers are stable for longer than the indicated guarantee period. However, MHC Pentamers, like any other protein based reagent, will lose their activity eventually. Unlabeled and biotin-labeled Pentamers may be stored for longer periods at -80°C. |
| Cells were fixed before staining with Pentamers | Pro5® Pentamers rely on engaging multiple MHC molecules with multiple TCRs on the cell surface during staining. In order for this to occur the TCRs need to be mobile in the cell surface during staining. As a consequence cells should only be fixed after Pentamer staining. |
| Stained cells are from an species or MHC or HLA mismatched donor | Pro5® MHC Pentamers will only work for cells that match the species and the HLA type of the Pentamers, although there may be exceptions in the context of allograft or xenograft rejection. |
Further Reading
Interrogating the repertoire: broadening the scope of peptide-MHC multimer analysis. Davis, MM et al Nature Immunology 2011 11:551 [PubMedID: 21760610]
Tracking epitope-specific T cells. Moon, JJ et al Nature Protocols 2009 4:565 [PubMedID: 19373228]
Tricks with tetramers: how to get the most from multimeric peptide-MHC. Wooldridge, L et al Immunology 2009 126:147 [PubMedID: 19125886]
Immunology of Diabetes Society T-Cell Workshop: HLA class I tetramer-directed epitope validation initiative T-Cell Workshop Report-HLA Class I Tetramer Validation Initiative. Mallone, R et al Diabetes/Metabolism Research and Reviews 2011 27:720 [PubMedID: 22069251]
Harmonization guidelines for HLA-peptide multimer assays derived from results of a large scale international proficiency panel of the Cancer Vaccine Consortium. Britten, CM et alCancer Immunology and Immunotherapy 2009 58:1701 [PubMedID: 19259668]
