Your basket is currently empty!
ProT2® MHC Class II Tetramers
ProT2® MHC Class II Tetramers
track antigen-specific CD4+ T cells with the
most comprehensive class II tetramer catalog range
now also available for H-2 IAb, IAd, IAg7
ProT2® MHC Class II Tetramer
Detect and separate single antigen-specific CD4+ T cells with our new best in class ProT2® MHC Class II Tetramers
ProT2® MHC Class II Tetramers allow you to detect single antigen-specific CD4+T cells accurately by flow cytometry. They can also be used to separate cells for culture, expansion and further study. ProT2® Tetramers enable you to study in depth CD4+ T cell immune responses in a wide range of disease areas including autoimmune disease, cancer and infectious diseases. ProT2® Tetramers are made by ProImmune’s experienced team who have been providing superior MHC reagents for more than 20 years, including our world-leading Pro5® MHC Class I Pentamers. Custom ProT2® Tetramers are available within approximately 6 weeks from ordering.
Features of ProT2® MHC Class II Tetramers
- From the most experienced commercial providers of MHC multimers
- Stringent purification and quality control in line with our ISO:9001 quality system
- Proven technology for detecting antigen-specific T cells
- Broadest range of catalog items available
- Flexible labelling options
- Guaranteed shelf life
- Industry leading customer service from immunologists who understand your work
Watch our presentation about ProT2 MHCII Tetramers
ProT2® staining for CD4+ T cell epitopes
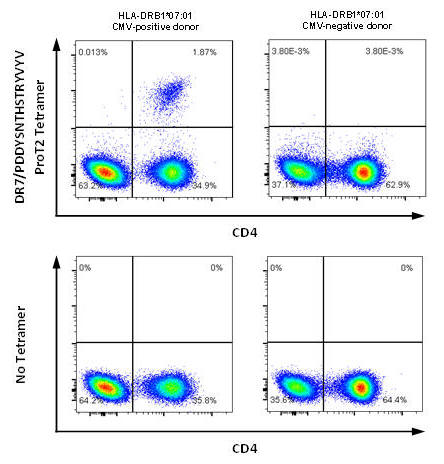
(A) Data for HLA-DR7/CMV from Prof Paul Moss group, University of Birmingham, UK
(B) Data for H-2 IAb/2W1S from Dr David Withers group, University of Birmingham, UK
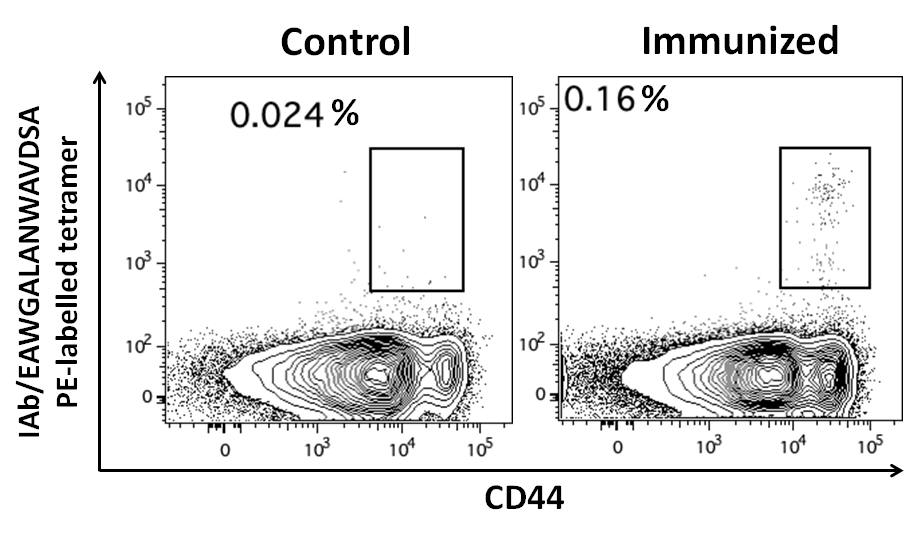
Figure 1. (A) PBMCs from CMV-positive and CMV-negative donors were stained with DRB1*07:01/PDDYSNTHSTRYVTV ProT2® Tetramer. Plots on the left show cell samples from a CMV-positive donor; plots on the right show cell samples from a CMV-negative donor. A population of CD4+ tetramer-positive cells is visible in the upper right quadrant of the plot from CMV-positive donor cells stained with ProT2® Tetramer. All plots were derived by gating on live, CD19-negative, CD14-negative, CD3-positive lymphocytes. (B) Splenocytes of control and 2W1S peptide immunized mice gated on live, CD3+,CD8- CD4+ events, stained with ProT2® H-2 IAb Tetramer.
Traditional functional assays for studying antigen-specific CD4+ T cells, such as proliferation or cytokine secretion, are not truly antigen-specific. The direct detection of antigen-specific CD4+ T cells has been difficult, due to the low frequency of these T cells (down to 1 in 100,000 white blood cells) and the low functional avidity, caused by low MHC/peptide-TCR affinity, reduced TCR clustering and coreceptor effects. The optimized manufacturing process for ProT2® Tetramers helps to address these issues by improving reproducibility and sensitivity of CD4+ T cell detection.
ProImmune provides ProT2® Class II MHC Tetramers with responsive, expert technical support and customer service. Contrary to ordering class II tetramers from in-house or non-commercial facilities, customers do not need to apply and wait for reagents or supply any peptide. ProT2® Class II MHC Tetramers are delivered quickly with stringent quality control and low batch-to-batch variability. As such ProT2® Class II MHC Tetramers complement ProImmune’s existing Pro5® MHC Class I Pentamer technology for detecting antigen-specific CD8+ T cells.
Key Publication
Kramer C. et al. (2020). “Generation and reactivity analysis of human recombinant monoclonal antibodies directed against epitopes on HLA-DR” AJT DOI: 10.1111/ajt.15950

Figure 2. HLA-DR-specific memory B cell clones isolated from peripheral blood using ProT2® MHC Class II Tetramers. A, Representative example of 3 independent experiments depicting the flow cytometry gating strategy to live single cell sort CD3-CD27+IgD-HLA-DR tetramer double positive B cells from PBMC. B, IgG antibody production by the clones was determined by ELISA. C, IgG positive clones were screened with HLA class II Lifecodes Lifescreen Deluxe kit to detect HLA antibody. The kit contains 5 groups of HLA class II beads and each data point represents a single bead group. D, HLA-specific B cell clones were tested with SAB assays to confirm tetramer specificity used for cell sorting. Each dot presents 1 clone and only MFI of bead with tetramer specificity is depicted. E, Percentage of sorted HLA-specific B cells from total memory B cells. F, Percentage of HLA antibody-producing B cell clones from sorted B cells. On the x-axis are the specificity of the tetramers used depicted.
Available Quantities
50 tests, 150 tests
Fluorescent Label
ProT2® MHC Class II Tetramers are delivered with a choice of standard R-PE or APC labels. Alternatively customers can purchase and conjugate our ProM2® MHC Class II Monomers to any other suitable avidin or streptavidin reagent.
Catalog ProT2® MHC Class II Tetramers
A broad range of MHC Class II complexes specific for a wide range of infectious diseases such as Influenza, CMV, hepatitis B and C, HIV, EBV, as well as cancer-related epitopes are available as ProT2® Tetramers. Catalog ProT2® Tetramers are despatched in 1-2 days for Stock items, and 3 weeks for Available items.
List of Catalog Class II Tetramers
Custom ProT2® MHC Class II Tetramers
Custom ProT2® Tetramers for your allele/peptide of interest are available within approximately 6 weeks from ordering. Peptide sequences are subject to prior evaluation by ProImmune for binding. Once a peptide sequence is accepted for custom synthesis we will synthesize both the peptide and MHC tetramer reagent.
Available Alleles
HLA-DR1 (DRB1*01:01); DR2 (DRB1*15:01); DR3 (DRB1*03:01); DR4 (DRB1*04:01); DRB1*04:02; DRB1*04:05; DR5 (DRB1*11:01); DR7 (DRB1*07:01); DR9 (DRB1*09:01); H-2 IAb; IAd; IAg7
If you are looking for an MHC allele not listed above, please contact us to enquire.
Fully compatible technology
ProT2® MHC Class II Tetramers can be used together with other Class II Tetramer reagents which customers may already have established in their labs.
ProT2® MHC Class II Tetramer staining
ProImmune has extensive experience in testing ProT2® MHC Class II Tetramers in-house in a variety of applications. This enables us to support our customers fully with their relevant application requirements, helping them to make best use of our technology.
ProT2® staining for CD4+ T cell epitope from Influenza
Data from ProImmune
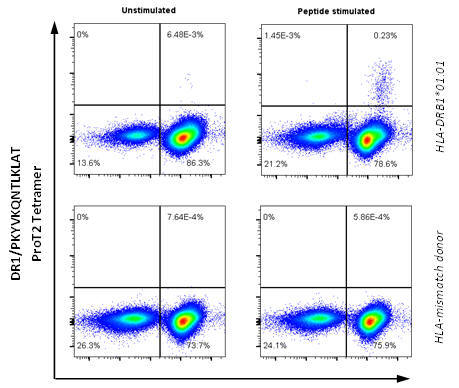
Figure 3. PBMCs from healthy donors were stimulated with peptide PKYVKQNTLKLAT (Influenza A HA 307‑319), cultured for 13 days, and stained with DRB1*01:01/PKYVKQNTLKLAT ProT2® Tetramer. Plots on the left shows a cell sample that has not been stimulated with peptide; plots on the right show the same cells that have been stimulated with peptide. A population of CD4+ tetramer-positive cells is visible in the upper right quadrant of the plot from peptide-stimulated HLA-DRB1*01:01 donor cells. All plots were derived by gating on live, CD19-negative lymphocytes.
ProT2® MHC Class II Tetramers may be used to study CD4+ T cell immune responses in infectious diseases such as CMV (see figure 1 above) and HCV, and in vaccine studies.
ProT2® staining for CD4+ T cell epitopes from HCV
Data from Dr Georg Lauer group, Massachusetts General Hospital, Boston
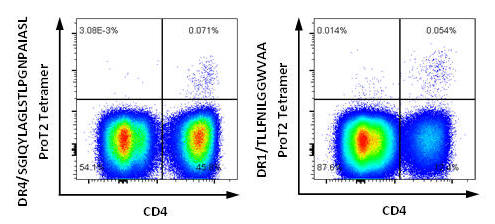
Figure 4. PBMCs from HCV-positive donors were stained with ProT2® Tetramer. The left plot shows a population of CD4+ tetramer-positive cells in the upper right quadrant from HCV-positive HLA-DRB1*04:01 donor cells stained with DRB1*04:01/SGIQYLAGLSTLPGNPAIASL ProT2® Tetramer. The right plot shows a population of CD4+ tetramer-positive cells in the upper right quadrant from HCV-positive HLA-DRB1*01:01 donor cells stained with DRB1*01:01/TLLFNILGGWVAA ProT2® Tetramer. All plots were derived by gating on live, CD19-negative, CD14-negative, CD3-positive lymphocytes.
ProT2® staining for CD4+ T cell epitopes from HCV
Data from Prof Ellie Barnes group, University of Oxford, UK
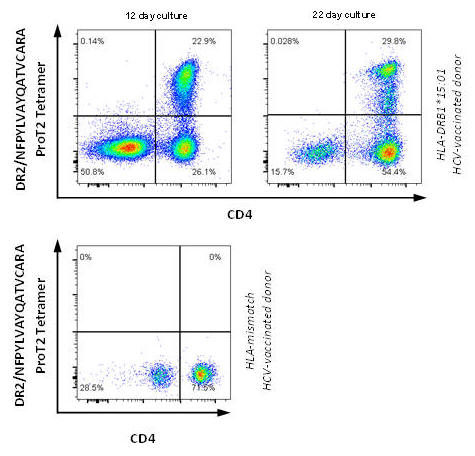
Figure 5. PBMCs from post HCV-vaccinated donors were stimulated with peptide NFPYLVAYQATVCARA and cultured. Cells were stained on day 12 and day 22 with DRB1*15:01/NFPYLVAYQATVCARA ProT2® Tetramer. A population of CD4+ tetramer-positive cells is visible in the upper right quadrant of the plots from HLA-DRB1*15:01 donor cells. All plots were derived by gating on live, CD3-positive lymphocytes.
To aid detection of low frequency populations that are beyond the limit of detection of standard direct Class II tetramer staining, expansion [1, 2] or enrichment with magnetic beads [3, 4] may be required; although some very low affinity antigens may not give detectable staining.
Sample Product Sheets
ProT2® MHC Class II Tetramer (APC Labeled)
ProT2® MHC Class II Tetramer (R-PE Labeled)
ProT2® MHC Class II Tetramer (Negative Control)
Key Publications of ProT2® MHC Class II Tetramers
Hartnell F. et al. (2020). Characterising HCV specific CD4+ T-cells following viral-vectored vaccination, directly acting anti-virals and spontaneous viral cure. https://doi.org/10.1002/hep.31160
Cianciotti B. et al. (2019). CD4+ memory stem T cells recognizing citrullinated epitopes are expanded in patients with rheumatoid arthritis and sensitive to tumor necrosis factor blockade. https://doi.org/10.1002/art.41157
Kramer C. et al. (2020). Generation and reactivity analysis of human recombinant monoclonal antibodies directed against epitopes on HLA-DR. https://doi.org/10.1111/ajt.15950
Gliwinski M. et al. (2020). Proinsulin-specific T regulatory cell may control immune responses in type 1 diabetes: implications for adoptive therapy.
References
[1] Novak et al. (1999). MHC class II tetramers identify peptide-specific human CD4(+) T cells proliferating in response to influenza A antigen. J Clin Invest. 104(12): R63-7. [PubMedID: 10606632].
[2] Novak et al. (2001). Tetramer-guided epitope mapping: rapid identification and characterization of immunodominant CD4+ T cell epitopes from complex antigens. J Immunol. 166(11): 6665-70. [PubMedID: 11359821].
[3] Day et al. (2003). Ex vivo analysis of human memory CD4 T cells specific for hepatitis C virus using MHC class II tetramers. J Clin Invest. 112(6): 831-42. [PubMedID: 12975468].
[4] Scriba et al. (2005). Ultrasensitive detection and phenotyping of CD4+ T cells with optimized HLA class II tetramer staining. J Immunol. 175(10): 6334-43. [PubMedID: 16272285].
