Your basket is currently empty!
Flow Cytometry
Pro5® Pentamers in Flow Cytometry
A major application for Pro5® MHC Class I Pentamers is in the detection of antigen-specific CD8+ T cells by flow cytometry. Pro5® Pentamers allow rare, peptide-specific CD8+ T cell responses to be investigated in great detail.
An alternative method of studying T cell responses is to use a limiting dilution assay (LDA), which involves culturing cells in the presence of antigen for several weeks in order to determine any responses. Using this technique it is not possible to determine the response of individual cytotoxic T lymphocytes (CTLs) without prior manipulation. Furthermore, many potential responding cells can not survive the lengthy assay period.
In contrast to this, by using Pro5® Pentamers, individual MHC-peptide-specific CTLs may be analyzed quantitatively on freshly isolated or previously frozen T cells. Following their isolation, the phenotype of these cells may be further characterized using techniques such as intracellular cytokine analysis, ELISpot or quantitative RT-PCR.
Example of Pro5® Pentamer Staining
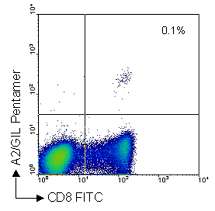
PBMCs from a healthy volunteer, seropositive for Influenza virus, were stained with Pro5® MHC Class I Pentamers. The plot gives a clear indication that 0.1% of live lymphocytes recognize the peptide epitope GILGFVFTL derived from one of the proteins of Influenza.
Labeling cells for flow cytometry involves using reagents that are specific to cellular markers, such as fluorescently labeled antibodies for detecting phenotypic cell surface molecules, and Pro5® Pentamers for detecting single antigen-specific CD8+ T cell receptors. The markers are typically cell surface proteins, although it is also possible to label intracellular proteins by first permeabilizing cells.
Many antibodies used in flow cytometry are directly conjugated to a fluorochrome. However, unlabeled and biotinylated primary antibodies can also be used in combination with a fluorescent-labeled secondary antibody. A single cell sample can be simultaneously analyzed for multiple markers using antibodies conjugated to different fluorescent labels. Ongoing advances in fluorochrome chemistry and cytometry instrumentation provide many flexible options for multi-parameter cell labeling and analysis.
